WRITTEN BY: HARRIE PHILLIPS
PGCertClinEd, BAdVocEd (VocEd&Trng), RVN, DipVN (Surgical, ECC), DipTAE (Development & Design), DipBus, TAA, MACE

The accuracy of any clinical pathology test is greatly determined by the quality of the blood sample collected from the patient. High-quality collection is classified as a “clean stick”, which means that the sample was collected after a single insertion into the vessel, and there were no issues with collecting blood, such as slow draws, issues finding the vein, vein ‘blowing’, or the patient moving a lot. These types of issues will increase the likelihood of the blood clotting or the occurrence of cellular damage.
Ideally, blood should be collected from the jugular, as this generally allows for better sampling. However, if a patient is known to have a coagulopathy (clotting dysfunction), blood samples should then be taken from the saphenous (either lateral or medial) vein or the cephalic vein. Blood collection can also be taken from the femoral, dorsal pedal, auricular, and essentially any spot on the body where a vessel can be seen.
Accurate and efficient blood collection comes down to being prepared and having practice at collecting samples on a wide variety of patients.
LET’S LOOK AT THE STEPS FOR COMPLETING A BLOOD DRAW,
REGARDLESS OF VEIN SELECTED
1. Prepare your equipment first
There is nothing worse than going to restrain a wriggle dog, get some blood then turn around to discover you are missing your blood tubes.
As tempting as it is to just grab a needle and syringe, some tubes and prep and go for it, some careful preparation will ensure you get the right volume of blood. The second worse thing is discovering you don’t have enough blood to fill the tubes!
As you are handling blood, you should be wearing examination gloves as a minimum level of PPE.
Blood Tubes
Work out what tubes you need for the test, if you’re unsure, the external pathology lab will have protocols for you to follow or a useful chart you can pop on the wall, just ask them.
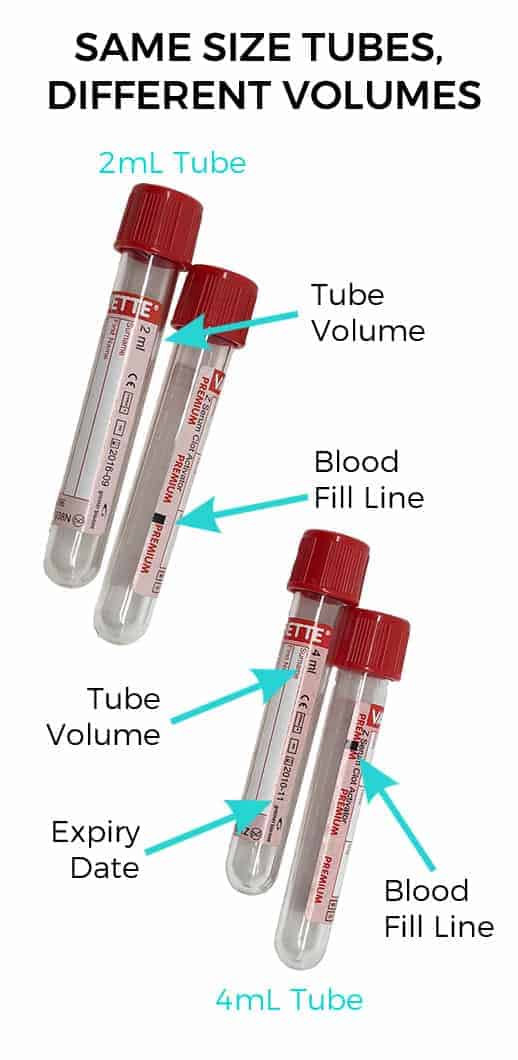
Once you have your tubes, check they are in date. Out of date tubes can affect results.
Next, work out the total volume of blood you need. Each tube requires a specific volume of blood. Paediatric tubes often only require 0.5 or 1mL of blood. Standard vacutainers generally need 2 or 4 mLs. The tube size can be the same, but the volume needed can differ. If you don’t put in enough blood, the lab may not have enough blood sample to run the tests your vet has requested. Too much blood means there is not the right level of anticoagulant or coagulant additive to be effective.
Needle & Syringe
Your total blood volume will then tell you what size syringe you need. If you have 3 x 2mL tubes, you know you need a 10mL syringe, and need to obtain at least 6 mLs of blood.
Needle size, you want to go as big as you can – considering the patient’s size and vein you have selected for venepuncture. If your syringe has a small gauge needle attached, you may end up damaging the blood cells, which can affect results. For medium or larger dogs a 20g needle is satisfactory, with a 22g on smaller patients.
2. Site preparation
It can be tempting to just wipe over some alcohol or a chlorhex/alcohol mix and then stick away with your needle. Especially when you’re confident you know where the vein is.
But remember, you’re passing an object from the very dirty skin straight into the venous system. You will push through some of that contamination and natural flora and fauna into the blood. For this reason, clipping the hair not only gives you better visualisation, it’s better patient care as well. You’re also likely to contaminate your sample if you don’t.
For routine blood draws, after clipping, you should still use a scrub based skin prep to remove gross contamination. The back and forth action of cleaning will help to remove the debris. Follow this with your alcohol or chlorhex/meths mix, wiping from the insertion site out, not top to bottom.
If you need to palpate for the vein, do this prior to performing your site preparation. If you need to palpate again after your site preparation, then ensure you wipe over with your chlorhex/meths again.
If you’re obtaining samples for a blood culture, then you will need to clip more hair than normal, and perform a full three step surgical scrub. You’ll also need to wear sterile gloves and keep your needles and syringes completely sterile as to not break asepsis.
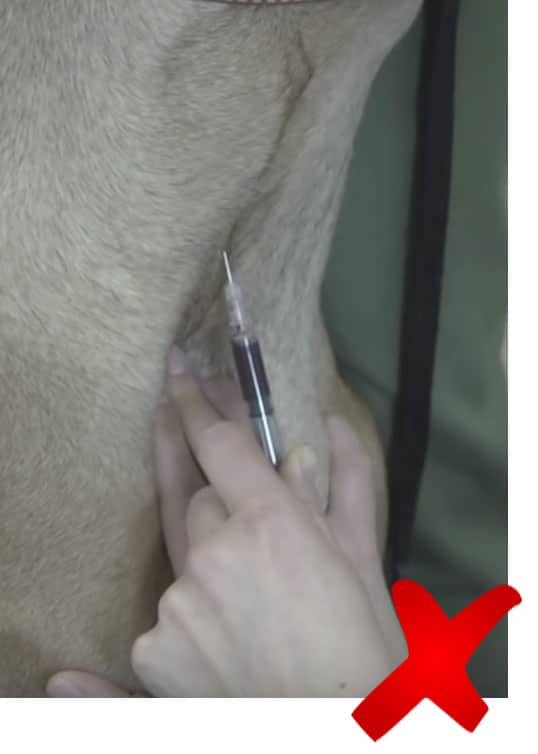
3. Obtain your sample.
Opening your needle and syringe aseptically, join them together then the bevel of the needle facing your volume graduations. Bevel up allows for a easier insertion, and seeing the volume markings allows you to ensure you have collected an appropriate sample amount.
Check that your plunger is moving freely.
Insert the needle at a 45o angle into the vein and apply gentle pressure on the plunger. Once blood is seen in the hub of the syringe, continue to gently draw the blood back until you have the required volume. Don’t pull it too fast as you can collapse the vein, slowing down blood flow.

4. Pop your blood in the tubes
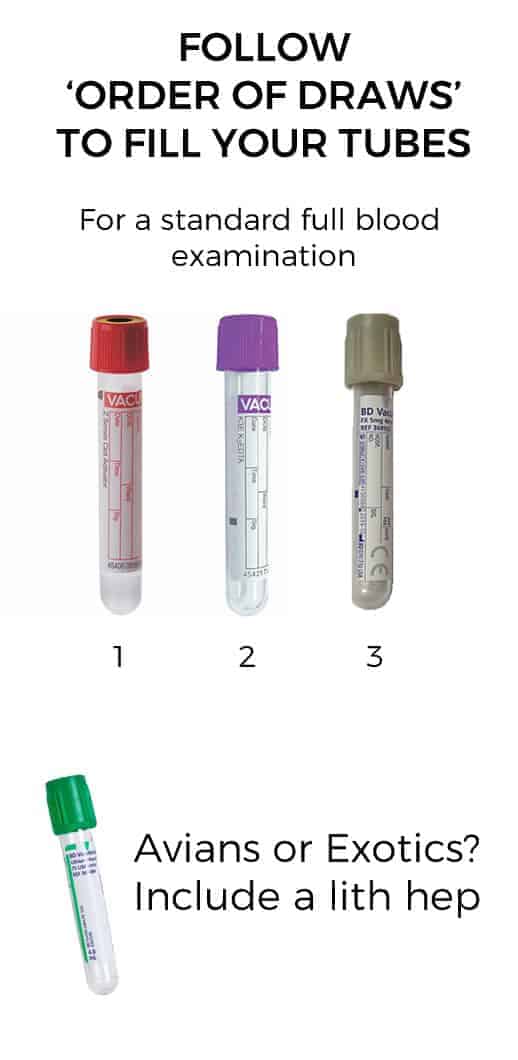
It’s common to see both vets and nurses reach for the EDTA (purple top in Aus) and fill that first. It’s true we do not want our blood sample to clot, however, we don’t want to contaminate our other tubes either. External labs will have posters for you with the ‘order of blood draw’ – this shows you the appropriate order to fill your tubes.
For a routine full blood examination, we use an EDTA (purple), a serum (red) and a sodium fluoxolate (grey) tube. The first tube to fill is actually the serum (red), then the EDTA, followed by the grey top. This ensures you do not accidentally contaminate the serum tube with EDTA.
If you have obtained a sample in a timely manner, then you have time to fill all the tubes and gently invert them before the blood will clot. Blood doesn’t clot normally until over a minute.
Remove the tube lid and your needle, then fill the tube without touching the tube sides. This is important to ensure we limit potential contamination. Place the appropriate blood volume into the tube by only filling to the ‘fill line’ then recap. Gently invert several times to ensure the additives have fully mixed with the blood.
For exotics and avian patients, you’ll send a lithium heparin tube (green top) and pop in a fresh blood smear as well. EDTA tubes are unsuitable as they can result in falsely elevated potassium/falsely depressed calcium readings.
Don’t inject your blood sample into the vacutainer tubes through the needle. This second pass through the needle is more likely to cause haemolysis of the blood, affecting the sample.
5. Label, label, label
Ensure the patient name, owner name, date of collection, and for some samples, the time, is recorded on the tube.
6. Wash your hands
Yes, you were wearing gloves, however, for your, and your patient’s safety, follow the five moments of hand hygiene and ensure you thoroughly wash your hands.
JUGULAR VENEPUNCTURE TECHNIQUE
want to learn more?

CLINICAL PATHOLOGY
SHORT COURSE
This course will ensure you have a good knowledge of laboratory techniques commonly used in the veterinary clinic. You’ll be confident in knowing what equipment is used and how to maintain it including microscopes, refractometers, in-house lab machines, centrifuges etc. We also cover how to perform various pathology procedures urinalysis including sediment, PCV/TP, faecal floats, blood smears, and snap tests etc. The foundations of haematology and biochemistry are also covered, along with the importance of quality assurance procedures. Setting up and assisting with various centesis procedures is also included. Most importantly, you’ll learn about the correct handling and storage of samples to ensure accurate test results.
Check it out